-

Clinical Research Services
We offer the most efficient clinical research services.
-

Digital Research Services
We offer the most agile development & digital services.
-

HealthSourcing
We provide skills in Clinical Development, Regulatory and Pharmaceutical Affairs, Quality Assurance...
Agile Clinical & Digital Research Organization
Agile : the ability to quickly deploy studies, manage complexity, and enhance quality in compliance with our regulatory environment
Go Beyond Expectations
Our objective is to exceed expectations
Operational Efficiency
We provide tools, knowledge and ressources
Smart Budgets
Quality and timing for an affordable price

We’ll Help You To Deliver Your Clinical Trial
Our flexibility and experience of the pharmaceutical and medical device market, combined to our cost-conscious approach makes us different from other CROs. With a single point of contact and the systematic implementation of back-ups, we are able to respond to the rapidly changing needs in terms of studies, while respecting delivery deadlines.
0
Years of Experience.
0
Happy Clients
0%
Satisfaction
Start A Good Clinical Plan
To maximize return on your R&D investments, strengthen your market position and efficiency, PopsiCube and our Project Managers should be involved as early as possible in the product lifecycle management to align expectations and fully understand the objectives of your development plan.
Moreover, PopsiCube will use leading-edge technologies like eTools and data analytic platforms highly contributing to your clinical plan efficiency.
Meet Our Leadership Team
Our diverse and experienced Board of Directors provides ongoing counsel and oversight while ensuring the company adds value and acts in the best interests of our clients.

Fabrice Beauchêne
Founder & Group ChairmanFabrice has over 20 years of experience in drug development for a US contract research organization (CRO) as well as for French and global pharmaceutical companies. Fabrice also acts as International Informatic Project Manager for a pharmaceutical firm in charge of the implementation of clinical e-Tools. In 2004, he launched POPSI CUBE, to carry out a variety of clinical development projects with large and small international pharmaceutical or medtech customers. Fabrice has a Mphil in Energetic Physiology and a Masters’ degree in statistics applied to medicine.

Sylvie Kahn
VP, WW Operations DirectorSylvie has more than 20 years of expertise in the pharmaceutical industry. She worked in laboratories and Contract Research Organisations (CRO) in clinical research with effective management of teams and individuals, both in matrix and structured environments. - International experience. - Gradual and regular promotions: CRA, Clinical Project Manager, GCP officer, Purchasing Manager, Methods & Training Manager, Head of Corporate Operations at Pharmacia (acquired by Pfizer).
They speak from experience…
News & Thoughts
Read the latest News and Announcements from PopsiCube
Launch of Astrum CRO
8 November 2023 We’re proud and very excited to introduce the brand new CRO, Astrum!Our strong and established European CRO’s Bioclever, Blueclinical, Pharmalog and Popsicube havejoined forces with the support of Henko Partners to form Astrum, a mid-size pan-European CRO.Astrum will provide a broad range of end-to-end clinical development services through a dynamicteam of highly […]
ADDITIV Médical France – 26 january 2021
Popsicube has participated to the virtual show dedicated to 3d printing in the medical field. For more information : https://www.additiv.events/medical-france?cid=bb68415b-ae2c-434a-a229-379aeb895955
Mission TEC will be present at the 1st edition of the Summer Health Campus
Mission TEC, a division of POPSICUBE, will be present on Thursday 6 and Friday 7 June 2019 at the 1st edition of the Summer Health Campus which will take place in Toulouse. On the schedule: conferences on the medicine of the future, the impact of sport on health as well as a debate on the […]
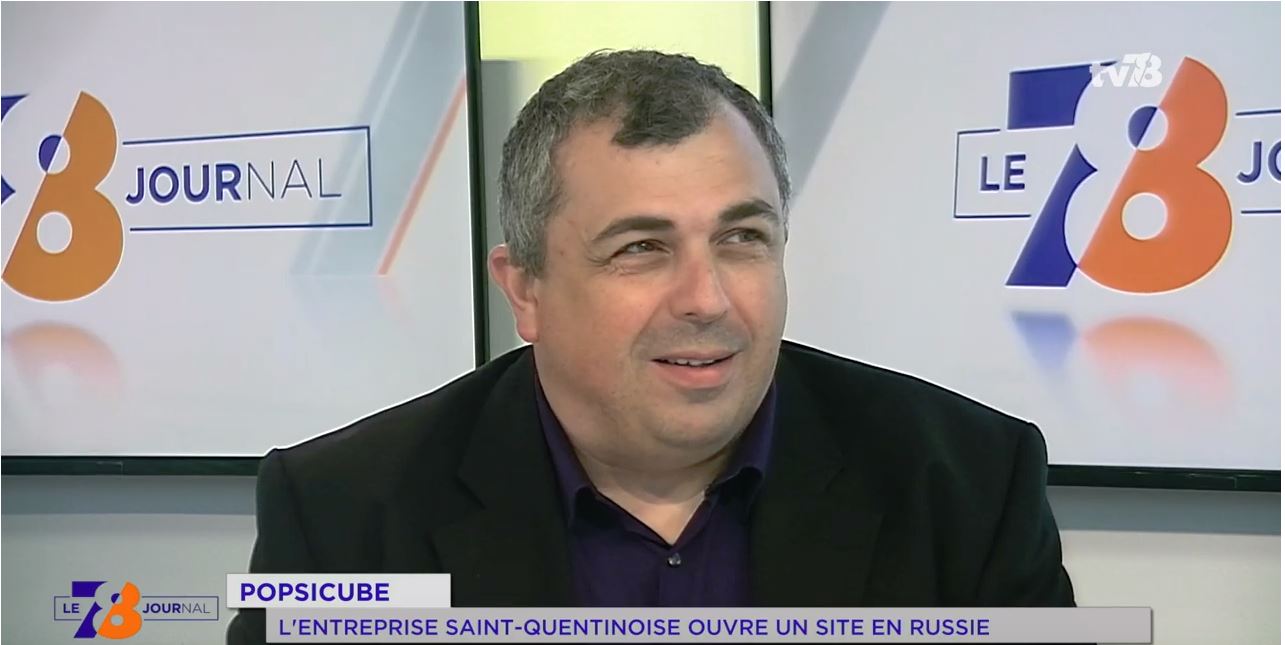
Discover Fabrice BEAUCHÊNE’s interview in the TV78 news
On 2019 April the 24th , M. Fabrice BEAUCHÊNE, Founder and Chairman of POPSICUBE, was invited by the Yvelines television channel TV78 to talk about the recent opening of a Russian affiliate to continue its international expansion. Credits : TV78 – La chaîne des Yvelines
Popsi Cube sponsor of the 2nd Kiev Clinical Research Forum 16-17 May 2019.
Come & meet us on our booth #16 at the MERCURE KYIV CONGRESS Address: V. Hetmana, 6, Kyiv, Ukraine, 03057 What is KIEV CLINICAL RESEARCH FORUM 2019? Kiev Clinical Research Forum is a global discussing platform for the clinical research industry based in Eastern Europe. The 2nd Forum follows a huge success of the inaugural […]
Popsi Cube sponsor of the 8th Day of Clinical Research next January 31
Come & meet us on our booth #22 at the Hyatt Regency Paris Etoile – Porte Maillot More information